Global CRO Drives Study Startup Efficiency by Converting Local IRB Sites to Central IRB Review
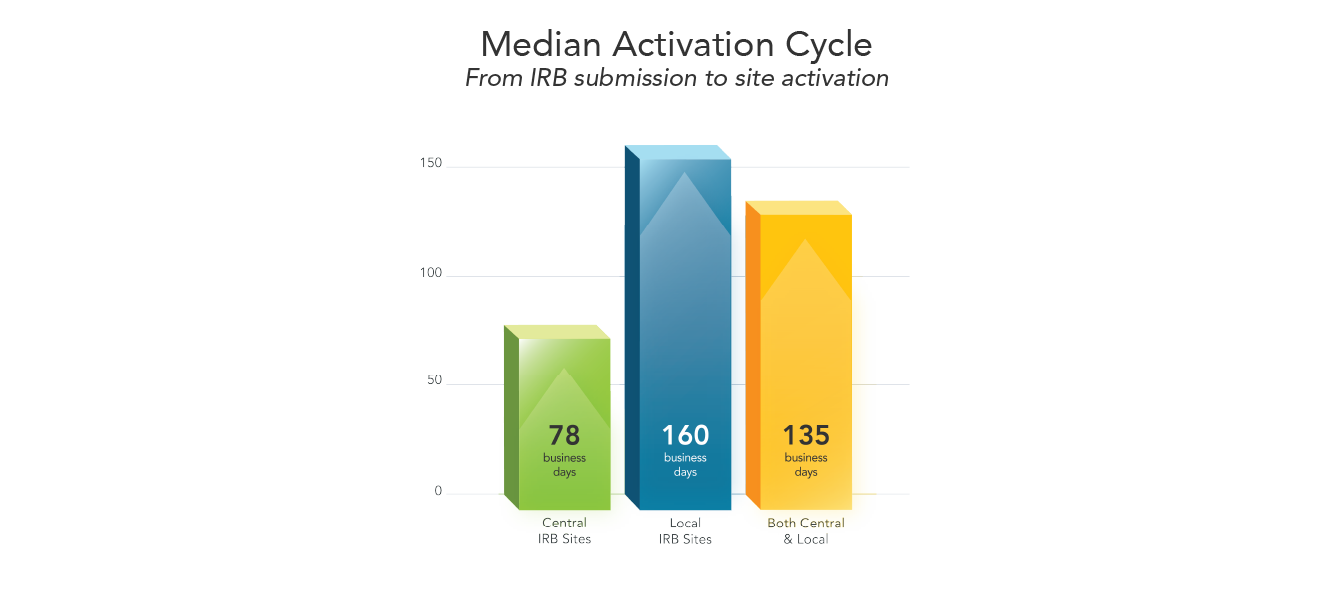
Research sites relying on a local institutional review board (IRB) typically take at least twice as long to activate than the sites relying on the study’s single central IRB. Local review can also decrease efficiencies throughout the study’s lifecycle.
In collaboration with Advarra, the global CRO substantially improved the ratio of sites able to use Advarra as the central IRB, which helped:
- Significantly reduce startup timelines
- Simplify site management
- Save money for the CRO's sponsor clients
Find out more in our case study.
DATE
Time: 1 p.m. ET, 12 p.m. CT, 10 a.m. PT
Duration: 1 hour
